
Jeremy Patrick
Laboratory Automation Systems Engineer
📍 Contact Information
Location: Los Angeles, CA
Email: jeremypatrick36@yahoo.com
Phone: (805) 630-8472
🎯 Recent Role
Position: Automation Engineer
Company: Capsida Biotherapeutics
Duration: May 2022 - June 2025
Status: Available Immediately
🚀 Executive Summary
Innovative Laboratory Automation Engineer with expertise in system design, assembly of lab automation robotics, and hardware integration. Develop and implement automation solutions that enhance laboratory efficiency and accuracy, ensuring seamless integration of robotic systems into existing laboratory workflows. Proven experience in system architecture, experimental design and system qualification, emerging technology integration, vendor negotiations, and capital expenditure management. Experienced in driving full lifecycle of projects from conceptualization to implementation while managing cross-functional teams and contractor relationships.
Professional Background & Experience
Automation Engineer - Capsida Biotherapeutics (2022-2025)
Led automation architecture for AAV engineering workflows in pharmaceutical research environment. Managed GitHub repositories, deployed high-throughput screening platforms, and developed automation systems that generated critical data supporting two FDA-approved IND applications for clinical trials. Ensured data integrity and traceability requirements for regulatory submissions.
NGS Lead Research & Development / Designated Automation Specialist - California Department of Public Health (2021-2022)
Led Next-Generation Sequencing research and development initiatives as designated automation specialist for automated nucleic acid extraction systems during COVID-19 testing expansion. Developed and validated whole genome enrichment workflows, pioneered automation protocols for high-throughput genomic analysis, and established quality control frameworks for public health genomic surveillance programs.
Senior Laboratory Technologist - PerkinElmer (2020-2022)
Performed high-throughput clinical testing in accredited laboratory environment. Mentored teams on automation systems, developed optimization scripts for resource management, and maintained strict compliance with clinical laboratory standards.
Clinical Laboratory Technician - Fulgent Genetics (2020)
Implemented Hamilton Microlab Prep automation systems, significantly increasing DNA extraction throughput.
🏥 Professional Engagement & Impact
SLAS Conference: Represented Capsida Biotherapeutics at SLAS conferences, presenting high-throughput screening platform innovations to the automation community. Active engagement with industry professionals and attendance at conferences to stay current with emerging technologies and trends.
Regulatory Success: Automation systems generated critical datasets supporting two FDA Investigational New Drug applications and CDPH epidemiological surveillance programs, demonstrating expertise across pharmaceutical and public health regulatory frameworks.
Engineering Solution 1: High-Throughput NGS Platform
Capsida Biotherapeutics needed to scale their AAV engineering capabilities to meet increasing demand for high-throughput screening. The existing manual NGS workflow was limited to 32 samples per run, creating bottlenecks in the drug development pipeline and limiting research capacity.
Design, integrate, and deploy a fully automated high-throughput NGS platform that could handle 384 samples per run while maintaining data integrity and sample quality. The system needed to incorporate solid phase extraction using pneumatic systems and integrate seamlessly with existing LIMS infrastructure.
🚨 Key Challenges
- Integration of pneumatic systems with liquid handling operations
- Reagent Fill Module Failures: Solenoid lifecycle determination required before production deployment
- Maintaining sample integrity through complex multistep workflows
- Ensuring data integrity across interconnected systems
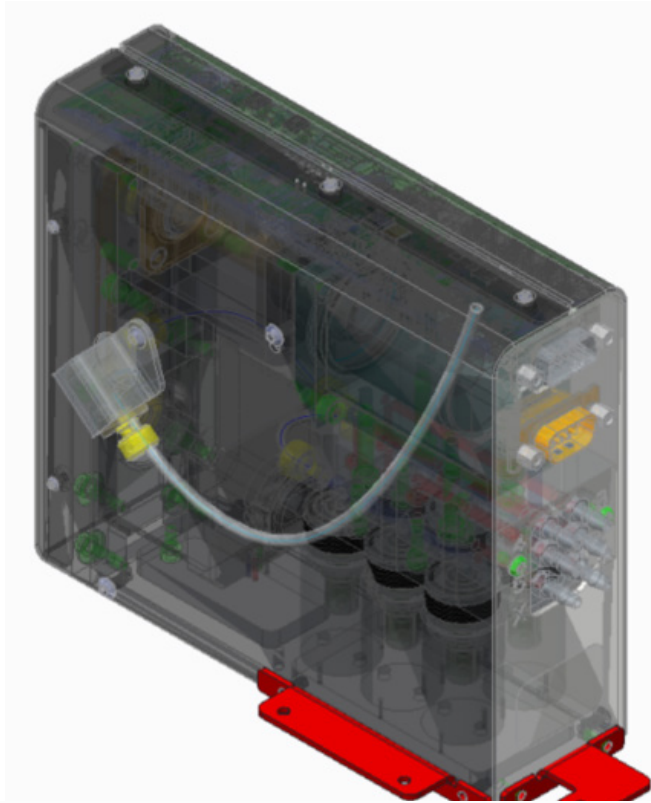
Reagent Fill Module
Fluidic component requiring solenoid lifecycle validation for reliable operation
🎯 Engineering Methodology
- Risk assessment and failure mode analysis
- Solenoid Lifecycle Validation: Systematic testing and characterization of reagent fill module components
- Stakeholder requirements gathering and process mapping
- Iterative design with continuous feedback loops
- Validation planning and regulatory pathway assessment
Engineering Solution 1: Actions & Results
- Systematic Design Architecture: Engineered pressure regulation system with triple redundancy and real-time monitoring
- Reagent Fill Module Optimization: Conducted comprehensive solenoid lifecycle testing and validation to prevent production failures
- Full End-to-End Automation: Eliminated operator intervention, removing ergonomic strain and ensuring consistent reproducibility across all process steps
- Integrated Quality Control: Established automated QC checkpoints with immediate failover protocols
- Robust Error Recovery: Designed comprehensive failover mechanisms ensuring 99.9% process continuity and operational efficiency
- 12x Throughput Increase: From 32 to 384 samples per run
- Complete Operator Independence: Eliminated manual intervention ensuring superior reproducibility and process continuity
- 65% Time Reduction: Compared to manual methods
- 99.5% Success Rate: With <1% sample failures
- 22% Cost Reduction: Through optimized reagent protocols and operational efficiency gains
🎥 Live Demonstration
Automation Pipeline in Action: View automated NGS workflow demonstration
🏆 Impact on Organization
This project transformed Capsida's research capabilities, enabling them to process significantly more samples with higher accuracy and lower costs. The automated platform became the foundation for their high-throughput screening operations, directly contributing to accelerated drug development timelines. The automation systems developed generated comprehensive datasets with full data integrity and traceability that were instrumental in two successful FDA Investigational New Drug (IND) applications, enabling advancement to clinical trials.
Regulatory Contribution: Platform designed to meet pharmaceutical data standards, ensuring complete traceability and quality control required for FDA submissions. Additional information available at capsida.com
Engineering Solution 2: AAV Vector Titer Assessment
AAV vector titer assessment was a critical bottleneck in the production pipeline. Manual ddPCR processes were prone to variability, with coefficient of variation reaching 15%, limiting batch sizes to 8 samples and requiring significant operator time for quality control.
Develop an automated AAV vector titer assessment system using ddPCR technology that would improve assay reproducibility, increase batch capacity, and reduce manual intervention while maintaining precision across diverse vector serotypes.
Problem Definition
Statistical analysis of manual process variability and identification of root causes
Design Philosophy
Single-use pathway strategy to eliminate cross-contamination risk
Validation Strategy
Comparative studies across multiple vector serotypes for robustness
Quality Framework
Multi-tier quality control with automated exception handling
Engineering Solution 2: Actions & Results
- Systematic Protocol Development: Developed specialized liquid handling protocols with built-in redundancy for viscous AAV samples
- Contamination Prevention Systems: Implemented comprehensive single-use pathway strategies with failover mechanisms
- Automated Quality Assurance: Established multi-tier QC checkpoints with automatic error detection and recovery
- Process Optimization Framework: Fine-tuned parameters with systematic validation across different vector serotypes
- Precision Improvement: CV reduced from 15% to 3.2%
- 4x Batch Increase: From 8 to 32 samples per batch
- Eliminated Cross-Contamination Risk: Through systematic single-use pathway implementation
- Multi-Serotype Validation: Proven performance across diverse vector types
Precision Excellence
Achieved industry-leading precision with CV <3.2%
Efficiency Gains
95% reduction in manual operator time
Multi-Serotype Validation
Proven performance across diverse vector types
Comprehensive Platform Validation: Precision vs Accuracy
📊 Outlier Analysis Results
DMSO Sample:
- Integra: 1.83% CV, 2.3% error
- Hamilton: 2.71% CV, 0.48% error
Aqueous Sample:
- Integra: 1.21% CV, 7.9% error
- Hamilton: 3.4% CV (with outlier), 2.63% CV (excluded), 6.8% error
Outlier: Channel 2 showed 10.2% CV vs 1.7-3.5% for other channels
🎯 Data-Driven Decision
- Systematic Analysis: Identified and characterized outlier performance
- Superior Accuracy: Hamilton achieved better target dilution accuracy
- DMSO Excellence: 5x better accuracy (0.48% vs 2.3% error)
- Holistic Evaluation: Performance + end-to-end automation capabilities
🧠 Systematic Validation Approach
Our comprehensive validation identified Channel 2 as a statistical outlier (10.2% CV vs 1.7-3.5% for other channels), demonstrating rigorous data quality analysis. Even including this outlier (3.4% CV), Hamilton demonstrated superior accuracy across both sample matrices, achieving 5x better accuracy for challenging DMSO samples. This data-driven analysis directly informed our strategic platform selection. We took full ownership of this critical automation decision, selecting Hamilton for its optimal balance of accuracy, reliability, and comprehensive end-to-end automation capabilities that transformed our entire workflow architecture.
Questions & Discussion
💡 Ready to Discuss
- Technical implementation details
- Specific automation challenges
- Team leadership experiences
- Future technology trends
🎯 Key Strengths
- End-to-end system integration
- Data-driven problem solving
- Cross-functional collaboration
- Continuous innovation mindset
🤝 Thank You
I'm excited about the opportunity to contribute to AstraZeneca's automation initiatives and help drive the next generation of laboratory efficiency and innovation. Looking forward to our discussion!
📞 Contact
Email: jeremypatrick36@yahoo.com
Phone: (805) 630-8472
🌟 Availability
Status: Immediately Available
Location: Los Angeles, CA
Transition: Recent completion of major automation project at Capsida